A_Bowers
Moderator
Over the past several months I have been discussing here and there what the effects an intercooler has on a turbocharged vehicle.
The main discussion I want to promote with this thread is UNDERSTANDING the definition and principles behind it.
THIS will not be a Brand X vs Brand Y debate.
We are trying to keep this as objective as possible.
To start with we need to understand what is Heat soak.
(Borrowing this definition from a Lotus Forum)
http://www.lotustalk.com/forums/f25/please-explain-heat-soak-50397/
This is from a Forum dedicated to GT500 Mustangs
http://www.fordgt500.com/forums/archive/index.php/t-12220.html
Now there is a distinct difference between "Heat Soak" as defined here and reaching the Thermal efficiency of an Intercooler. We all know that the OEM Intercooler is horribly undersized, and very inefficient.
It heats very quickly at idle (Heat Soak). This is due to a few things.
1. It has very little density or material. And that material is very thin.
This leads to a very quick transition of temperatures. Stop and go traffic comes to mind. Those of us on tuned vehicles and even stock vehicles can appreciate this example. We have all experienced that sharp drop off in power and major surge once the turbo boosts more to compensate for a massive temperature spike.
2. Once rolling the intercooler can cool, but to an extent. This is where FPI or fins per inch comes into play.
Yes, technically the OEM intercooler cools, however it does not cool enough for most of our liking. It reaches a plateau of cooling efficiency due to FPI, materials used and physical size (get to that in a minute).
As a very poor example, think of it this way. You have one fan....it spins to its maximum cooling you off. Thats great...now add 2 fans.....it cools you off even more.
Why? You have more cooling capacity....to translate it over to our discussion its an extra set of fins in an intercooler. Hope that is clear enough and gets my point across.
3. Size. The OEM unit is small...undersized almost. 1 inch and some change across and doesnt have a very good FPI measurement.
This unit plateau's off in its cooling efficiency once the RPMS start to rise. You are compressing more and more air, spinning the turbo faster and faster as the RPMS rise, so therefore more heat is generated.
I will try and dig up some logs of OEM intercooler logs (if i can find them...Petitioning readers of this post to chime in with logs) showing the rise in temps in proportion to a rise in RPMS.
This is not unusual and actually expected from the OEM unit.
An aftermarket unit from Company X, Y or Z will do a better job at cooling over the OEM one simply because of size and materials....this is not to say you can slap on any unit and say, hey ive got an intercooler therefore I make 45 more lbft of torque.
There are major design criteria that go into a unit. Density, materials, endtank design, pressure drop across the unit, bar and plate vs tube style.
As others have pointed out in prior conversations, Intercoolers in and of themselves do not produce more power as a downpipe or tune would, they simply maximize the ability to cool the intake charge, and that is where the power is made. It was already there, just being dissipated as heat, rather than power made to the ground.
This goes back to a law of physics. Energy cannot be created or destroyed, it simply changes states.
I am not an engineer by trade, however I have spent a good while around practical/industrial applications of heat exchangers (simple definition of an Intercooler) for several years now.
Im hoping that this thread shines some light on what the defined version of Heat soak is vs. the internet version of it is.
There are a few people (you know who you are), whom I am hoping will chime in with some hardcore science/engineer techno-lingo and formulas to solidify this discussion.
Thanks for the read guys, and hope it helps.
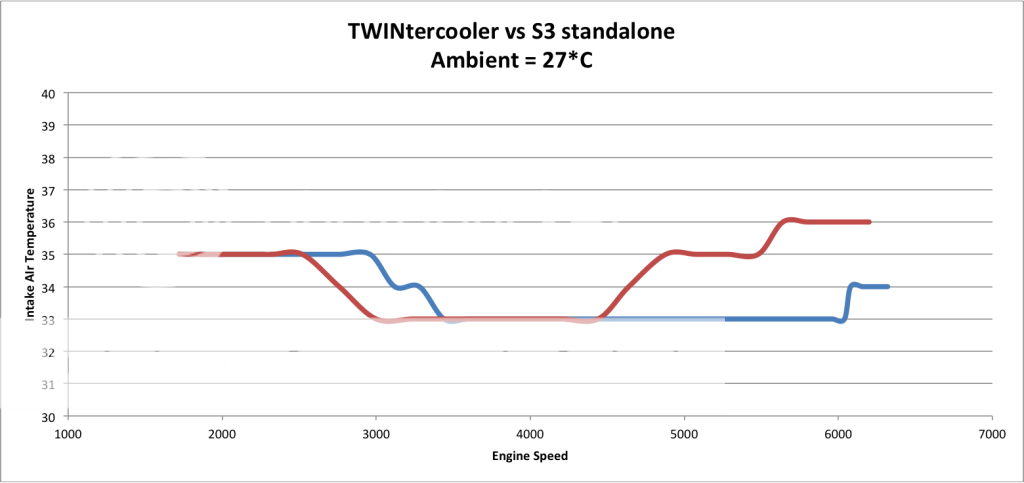
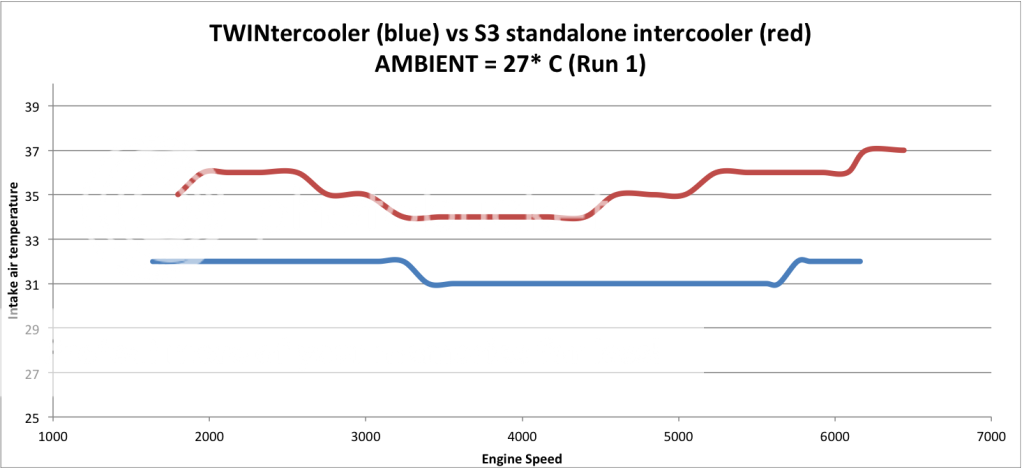
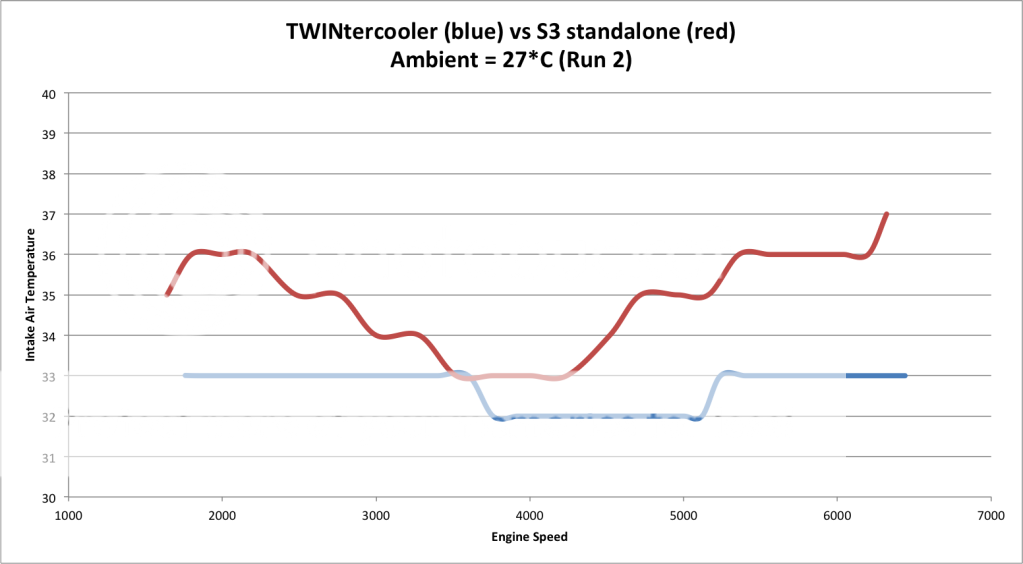
This is showing an aftermarket IC shown in blue as your RPMS rise on a 4th gear pull.
See in reference the other lines are actual boost and specified boost.
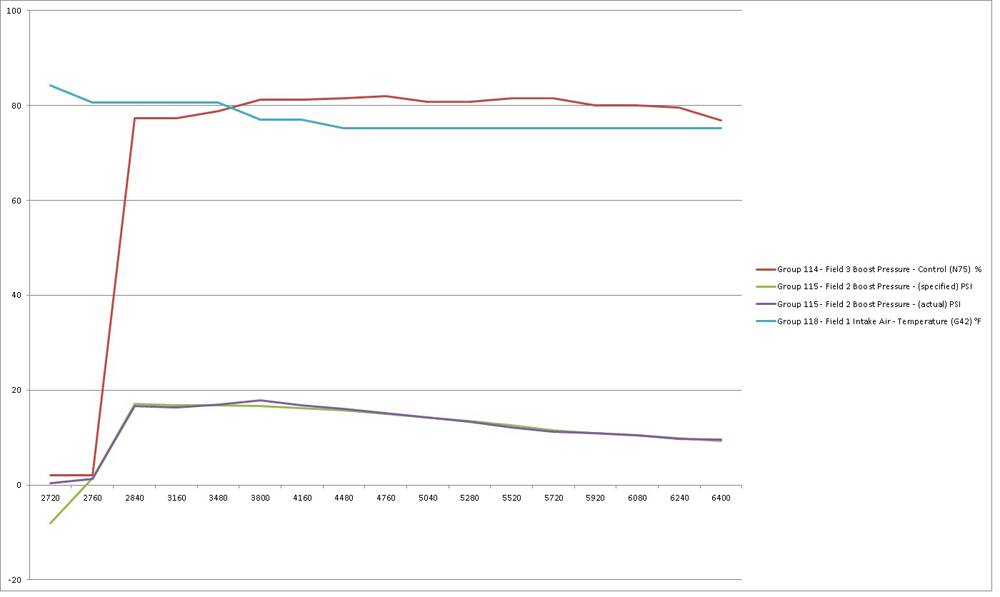
Pulled from MKV727's thread (this is for reference on the OEM S3 intercooler.)
This is the closest to our OEM intercooler as we can get, that I know that logs exist for. Pay close attention to the red lines (S3 unit) as the RPMS rise.
The main discussion I want to promote with this thread is UNDERSTANDING the definition and principles behind it.
THIS will not be a Brand X vs Brand Y debate.
We are trying to keep this as objective as possible.
To start with we need to understand what is Heat soak.
(Borrowing this definition from a Lotus Forum)
http://www.lotustalk.com/forums/f25/please-explain-heat-soak-50397/
Simple terms - a hunk of metal (or other thing) starts out at ambient temperature. It's exposed to other another temperature that warms (or cools) the surface. As time goes on, the head (or cold) evens out throughout the metal and the thing is "heat soaked". If you take away the source of the heat (or cold), the part will remain hot (or cold) for quite some time as it gives off the heat (or cold) that has "soaked" in the metal.
In terms of intercoolers, as the compressed air is compressed, it gets warmer. As it passes through the intercooler, the air gives off heat to the intercooler. And the intercooler gives off that air to the outside air that passes over the intercooler. After a while, the intercooler "heat soaks" and can't absorb as much heat from the compressed air to cool it. The temperature of the compressed air to the engine goes up and that increases the chance of detonation in the cylinder.
Sitting idle (with no air passing over the cooler) can also raise the "heat soak" causing loss of performance (or detonation), but it will reduce the "heat soak" as the car is moving with fresh (relatively) cool air passing over the intercooler again.
The same thing happens with cooling systems. The combustion chamber temperatures can get quite high, and the coolant passing through the cylinder head carries away the heat. But when you turn off the engine, there is still a high amount of residual heat in the chamber material that has not been removed. That heat "soaks" the head (and the coolant) and makes localized hot spots - the normal coolant temperature may be 195 degrees, but that local hot spot can easily get to be 20 - 50 degrees hotter. That's why many cars have a fan that will kick on after the car is parked if it detect that the engine is too hot. The Elise/Exige has a small pump that is turned on and cycles the coolant in the head through the heater circuit to dissipate the heat.
This is from a Forum dedicated to GT500 Mustangs
http://www.fordgt500.com/forums/archive/index.php/t-12220.html
I have had a lot of people inquire and ask about why there is such a difference with a Revan Racing dual fan heat exchanger versus heat exchangers with out fans.
The answer is in ambient and convective heat soak from the heat generated within the engine block.
When our cars are cold and we start them up and get going they are crisp and have tons of power because they are obviously cool. So what happens to many of us is we drive the car after it has gotten up to operating temperature and then we park the car. Whether you run in for a sandwich or whatever the car is sitting in a parking lot or your driveway after being warmed up and the operating temperature of the car is likely close to 180-200 plus degrees. When you let the car sit all of the heat from the block permeates the engine and subsequently the intercooler and the fluids within the intercooler and radiator causing those fluids to rise significantly. So an 30 minutes, an hour or two hours later you fire the car back up and it's completely heat soaked because nothing has been done to reduce ambient temperature because of the convective and ambient heat of the engine permeating and heat soaking the cooling mediums within the engine components.
When you utilize my dual fan heat exchanger the fans kick on and start to pull the heat from the heat exchanging and intercooling system immediately. Without fans you will have to drive the car for a good amount of time in order for the air to flow over the heat exchanger and cool the intercooler/heat exchanger fluid down to a point where timing is not pulled if you can get it to that point. Usually a long cruise on the highway can remedy the problem however stop and go traffic is brutal and difficult reduce the ambient temperature after a re-start.
The concept of the dual fans is that after the car has sat and gets heat soaked from sitting you immediately begin the process of removing the heat which gets you back into the power band much faster and gets the timing and spark advance retard point back to a point where it is under control and allows the ECU to provide you with maximum timing and spark advance.
In multiple data logs I have seen that the recover time of the intercooler fluid is much greater with the fans versus a non-fan heat exchanger. This is even faster when distilled water is utilized versus anti-freeze which is designe to retain heat, thus the reason why it is called 'Anti-Freeze', it holds heat.
A lot of people have said that I don't 'Race' my car. You don't have to 'race' your car to experience and utilize the benefits of a Revan Racing Dual Fan Heat Exchanger even if it is a daily driver.
Get the heat out as soon as possible and as quickly as possible.
Many drag racers use my heat exchanger with great success when they run back to back to back runs in something like True Street. For example, a member on SVT Performance, Kostas, actually improved his ET after each run at the NMRA event at Bradenton. His driving was good and his car wasn't pulling timing even as he went through the waiting time between rounds when you are not allowed to open your hood. And he doesn't have a trunk reservoir where you can access the trunk in a true street event. If he had no fans on the heat exchanger the idle temperatures would accelerate and his ET's would have been lower due to heat soak.
Please feel free to post your questions and I look forward to answering them.
Thanks
Now there is a distinct difference between "Heat Soak" as defined here and reaching the Thermal efficiency of an Intercooler. We all know that the OEM Intercooler is horribly undersized, and very inefficient.
It heats very quickly at idle (Heat Soak). This is due to a few things.
1. It has very little density or material. And that material is very thin.
This leads to a very quick transition of temperatures. Stop and go traffic comes to mind. Those of us on tuned vehicles and even stock vehicles can appreciate this example. We have all experienced that sharp drop off in power and major surge once the turbo boosts more to compensate for a massive temperature spike.
2. Once rolling the intercooler can cool, but to an extent. This is where FPI or fins per inch comes into play.
Yes, technically the OEM intercooler cools, however it does not cool enough for most of our liking. It reaches a plateau of cooling efficiency due to FPI, materials used and physical size (get to that in a minute).
As a very poor example, think of it this way. You have one fan....it spins to its maximum cooling you off. Thats great...now add 2 fans.....it cools you off even more.
Why? You have more cooling capacity....to translate it over to our discussion its an extra set of fins in an intercooler. Hope that is clear enough and gets my point across.
3. Size. The OEM unit is small...undersized almost. 1 inch and some change across and doesnt have a very good FPI measurement.
This unit plateau's off in its cooling efficiency once the RPMS start to rise. You are compressing more and more air, spinning the turbo faster and faster as the RPMS rise, so therefore more heat is generated.
I will try and dig up some logs of OEM intercooler logs (if i can find them...Petitioning readers of this post to chime in with logs) showing the rise in temps in proportion to a rise in RPMS.
This is not unusual and actually expected from the OEM unit.
An aftermarket unit from Company X, Y or Z will do a better job at cooling over the OEM one simply because of size and materials....this is not to say you can slap on any unit and say, hey ive got an intercooler therefore I make 45 more lbft of torque.
There are major design criteria that go into a unit. Density, materials, endtank design, pressure drop across the unit, bar and plate vs tube style.
As others have pointed out in prior conversations, Intercoolers in and of themselves do not produce more power as a downpipe or tune would, they simply maximize the ability to cool the intake charge, and that is where the power is made. It was already there, just being dissipated as heat, rather than power made to the ground.
This goes back to a law of physics. Energy cannot be created or destroyed, it simply changes states.
I am not an engineer by trade, however I have spent a good while around practical/industrial applications of heat exchangers (simple definition of an Intercooler) for several years now.
Im hoping that this thread shines some light on what the defined version of Heat soak is vs. the internet version of it is.
There are a few people (you know who you are), whom I am hoping will chime in with some hardcore science/engineer techno-lingo and formulas to solidify this discussion.
Thanks for the read guys, and hope it helps.
This is showing an aftermarket IC shown in blue as your RPMS rise on a 4th gear pull.
See in reference the other lines are actual boost and specified boost.

Pulled from MKV727's thread (this is for reference on the OEM S3 intercooler.)
This is the closest to our OEM intercooler as we can get, that I know that logs exist for. Pay close attention to the red lines (S3 unit) as the RPMS rise.



Last edited: